- GH aka somatotrophin
- produced by somatotropes (adenohypophysis)
- plasma half life 20-50mins
2. Structure of GH
- single polypeptide hormone
- 191 aa
- 2 disulphide linkages
- MW of 22kDa
- water soluble
- GH, prolactin, chorionic somatomammotropin have similar sequence homology
3. GH receptor
- MW of 70kDa
- GH binds to a cell surface receptor -> dimerisation of 2 GH receptors -> forms a dimeric complex -> activates tyrosine kinase and phosphrylation of the receptor and protein kinase on tyrosine residues
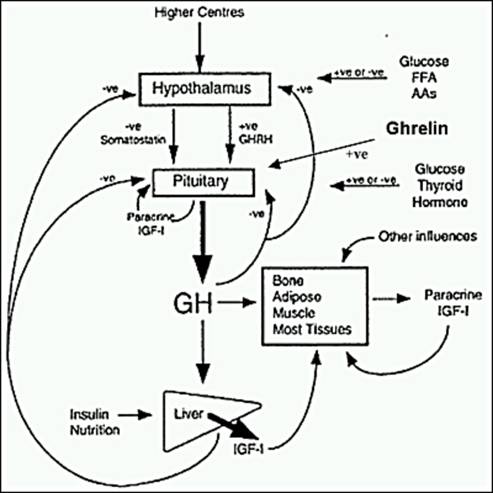
4. Regulation of GH secretion
GHRH from hypothalamus and GHIH from hypothalamus and D cells of pancreas.
5. Physiological and biochemical actions of GH
- Growth
- Direct effects are the result of growth hormone binding its receptor on target cells. Fat cells (adipocytes), for example, have growth hormone receptors, and growth hormone stimulates them to break down triglyceride and supresses their ability to take up and accumulate circulating lipids.
- INDIRECT: mediated by IGF
- stimulates cartilage grwoth
- stimulates linear bone growth by its action on the epiphyseal growth plates of long bones
- Width of bone also increases
- Normal carbs, lipid, protein and mineral metabolism
- Protein synthesis: GH increases the transport of AA into muscle cells and increase protein synthesis; increases synthesis of RNA and DNA in some tissue; resemble effects of insulin
- Carb metabolism: antagonises effect of insulin;decreased tissue glucose uptake and decreased rate of glycolysis; increased glycogen synthesis in the liver; increases hepatic glucose production via gluconeogenesis; prolonged administration can lead to dm
- Lipid metabolism: promotes lipolysis- increases release of FFA and glycerol from adipose tissue; increases circulating FFA, causes increased oxidation of FFA in liver (ketogenic): encourages use of fat as fuel and conserves glucose
- energy metabolism: GH increases the availability of fatty acids, which are oxidised as energy (spares glucose and proteins)
- Its effects on tissue/ organs
- adipose tissue: GH stimulates lipolysis -> breakdown of TG releases FFA and glycerol into the blood-> reduced synthesis of TG in fat cells
- muscle: GH stimulates lipolysis -> increase FFA in blood surrounding muscle and hence they will be used as fuels, conserving glucose and spares proteins -> since glucose uptake is reduced, rate of glycolysis is reduced; GH increases transport of AA into muscle cells and increase protein synthesis
- liver: stimulate production and release of IGF; GH increases oxidation of FA to acetyl CoA, enhancing ketogenesis; increased glycerol reaching the liver from lipolysis -> gluconeogenesis; increase glycogen synthesis in liver; glycolytic pathways are suppressed
- Mineral metabolism: promotes positive calcium, magnesium and phosphate balance; retention of Na+, K+ and Cl-
- As it binds to lactogenic receptors, it can stimulate mammary gland eg lactogenesis (prolactin like effects)
- Hyposecretion
- GH deficient dwarfs : Low GH and responds to exogenous GH
- Pygmies : Normal GH, but low IGF-1; post GH receptor defect
- Laron type dwarfs : High GH but low IGF-1 and IGF-2; lack functional hepatic GH receptor
- fail to increase GH levels in response to hypoglycaemia
- Hypersecretion
- most often due to pituitary tumour
- Children- gigantism - before epiphyseal plates close
- Adults- acromegaly - acral bone growth causes protruded jaw, enlarged nose, hands, feet and skull
- fail to suppress GH levels in response to glucose administration